

Break Through the Uncertainty
The CERTAS® Plus Programmable Valve is designed to minimize unintended setting changes from magnetic interference, protecting the valve from unintended setting changes due to everyday magnets¹ ² ³ * and 3T MRI machines 4,†

 KNOWN Risk
KNOWN Risk
- CERTAS Plus Programmable Valves resis the magnetic forces of 3T MRI machines. 4t
- Case Study: The Technical Abstract shows that wen 30 CERTAS Plus Valves were tested at 10 different MRI exposures, creating 300 opportunities, no unitentional setting changes were observed.
- MR Conditional: Non-clinical testing demonstrated that the CODMAN Certas Plus Programmable Valve is MR conditional at 1.5T and 3T.
 UNKNOWN Risk
UNKNOWN Risk
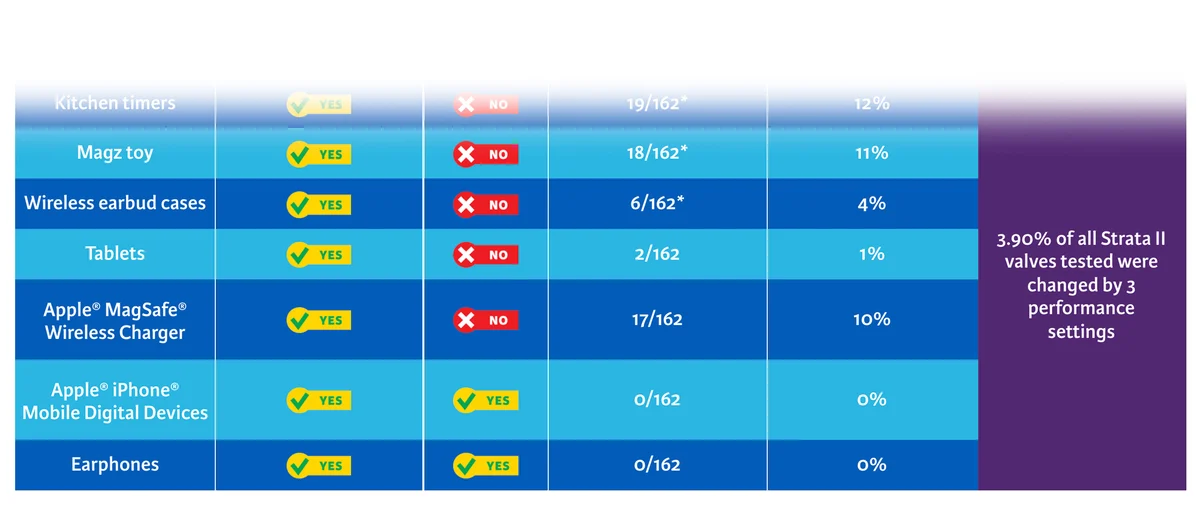
- Limit uncertainty from the risks of magnetic interference due to everyday magnets and MRI scanners. 1,2,3
- Explore the types of unsuspecting everyday magnets that may cause unintended setting changes that have been extensively tested by Integra LifeSciences.
- Apple iPhone Mobile Digital Device
- Magnetic Purse Clasps
- Magformers Toy
- Cochlear Implants
- Magnetic Phone and Tablet Cases
- Apple MagSAFE Wireless Charger, Headphones and Ear Buds
- Tablets
- Magnetic Wands
- Kitchen Timer
- Magnetic Tablet Cases
- Magz Toy
In a bench study, CODMAN CERTAS® Plus Programmable Valves resisted interference from common household magnets at close proximity (<5 mm) better than Strata II® Programmable Valves.
- 7 settings ranging from 25mm H2O to 215mm H2O to optimize shunt performance.
- Additional 8th setting, known as 'Virtual Off,' with a minimum operation pressure greater than 400m H2O
- The CERTAS Plus Valve can be placed anywhere as long as the tissue thickness is less than 10mm-including the occipital, frontal, retroauricular and subclavicular positions.
- The valve can be placed in any position of the body and will not impact the functionality of the valve's operation pressure of the SIPHONGUARD device.

- Enables clinicians to noninvasively turn off the valve versus doing a surgical ligation to limit flow
- 8th setting delivers a "Virtual Off" with a maximum operation pressure greater than 400mm H2O

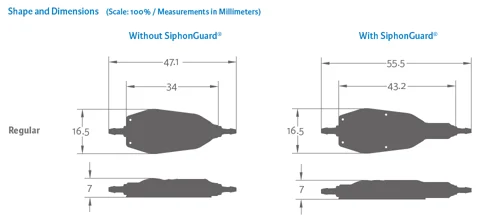
Certas Plus Regular
Without SiphonGuard
82-8800PL (Valve Only)
82-8801PL (Valve System)
82-8802PL (Valve System Unitized)
82-8803PL (Valve System Unitized with Bactiseal)
With SiphonGuard
82-8804PL (Valve Only)
82-8805PL (Valve System)
82-8806PL (Valve System Unitized)
82-8807PL (Valve System Unitized with Bactiseal)
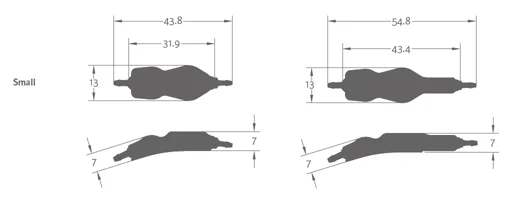
Certas Plus Small
Without SiphonGuard
82-8810PL (Valve Only)
82-8811PL (Valve System)
82-8813PL (Valve System Unitized with Bactiseal)
With SiphonGuard
82-8814PL (Valve Only)
82-8815PL (Valve System)
82-8817PL (Valve System Unitized with Bactiseal)

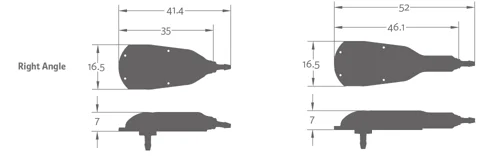
Certas Plus Right Angle
Without SiphonGuard
82-8820PL (Valve Only)
82-8821PL (Valve System)
82-8823PL (Valve System Unitized with Bactiseal)
With SiphonGuard
82-8824PL (Valve Only)
82-8825PL (Valve System)
82-8827PL (Valve System Unitized with Bactiseal)

Certas Plus Electronic Tool Kit
- Locator tools provides clear and easy-to-follow visual guidance to locate the valve.
- Locating dot provides instant feedback and shortens procedure time.
- Tactile feedback during adjustment for more confidence in setting changes
82-8852 (Electronic Tool Kit)

Certas Plus Tool Kit (Manual)
- Includes 2 locator tools for different tissue thickness.
- Allows for noninvasive reading to assist in monitoring and adjusting the valve pressure.
82-8851 (Tool Kit)
CERTAS Plus Value-Based Guarantee
If a patient experiences an unintended valve setting change in their implanted CERTAS Plus valve, then Integra will rebate the institutional purchaser $3000.*

For complete details on the CERTAS Plus Value-Based Guarantee, please see Program Description. Terms are subject to change or discontinuation without notice. Integra LifeSciences reserves the right to determine the sufficiency of any claim and compliance with program requirements.
Program Description
If an HCP with an NPI number who is pre-certified in the proper use of the CERTAS Tool Kit/CERTAS Plus Electronic Tool Kit according to the manufacturer's Instructions for Use has a patient who experiences an unintended valve setting change in their implanted CERTAS Plus Programmable Valve, then Integra will rebate the institutional purchaser $3000.
If you have any questions about coverage, or to initiate a claim, please contact your Integra account manager.
Please contact your Integra Account Manager to enroll in the program.

Hydrocephalus Resource Center
Explore instructional videos, IFUs and product information all in one simple and easy-to-access location.

Hydrocephalus Resource Center
The Hydrocephalus Association is an invaluable resource for the hydrocephalus community. From education to research, community network groups to patient advocacy, the hydrocephalus association is committed to finding a cure for hydrocephalus and to improve the lives of those impacted by the condition.

Understanding Hydrocephalus
This handbook is designed to provide basic information regarding the use of Codman hydrocephalus valves, to be used in conjunction with conversation with your doctor, as you navigate treatment options for hydrocephalus.
‡ References: 1. 2. 3. 4. 5.
Benchtop studies are not necessarily indicative of clinical performance.
T=Tesla.
* Testing included 6 samples of each valve, tested 162 times per product at a distance of 4.3 mm on average.
† Clinician should confirm valve setting after an MRI procedure.
The content of this document is not supplied or approved by Apple, Inc.
References:
- Data on file. Jacobs Institute Engineering Solutions. Hydrocephalus Shunt Valve Assessment. February 5, 2019. Integra LifeSciences, Plainsboro, NJ, USA.
- Data on file. Jacobs Institute Engineering Solutions. Hydrocephalus Shunt Valve Assessment. Oct. 16, 2019. Integra LifeSciences, Plainsboro, NJ, USA.
- Data on file. Jacobs Institute Engineering Solutions. Magnetic Influence of CHPV, CERTAS, and Strata II Shunt Valves. September 16, 2021. Integra LifeSciences, Plainsboro, NJ, USA.
- Data on file. Resistance of the CODMAN CERTAS® Plus Programmable Valve to Unintended Setting Changes When Exposed to a 3 Tesla MRI. February 2016. Integra LifeSciences. Plainsboro, NJ, USA.
- Products tested in study: Apple® AirPods® case; Apple® iPad Pro® 10.5-inch; Apple® iPhone® 5s; Apple® EarPods® with 3.5 mm Headphone Plug; Apple® iPhone® 12 Mobile Digital Devices; Apple® MagSafe® Wireless Chargers; Mini Skater 24 Sets Magnetic Button Clasp Snaps—Purses, Bags, Clothes; iPhone® 6 Wallet Case, Crosspace iPhone® 6s Envelope Flip Handbag Shell Women Wallet PU Leather Magnetic Folio Cover Cases with Credit Card ID Holders Wrist Strap for Apple® iPhone® 6/6s 4.7-inch Black; Dowling Magnets® DO-SS75 Magnet Mania Kit; Magformers® Classic (30 pieces) Set Magnetic Building Blocks, Educational Magnetic Tiles Kit, Magnetic Construction STEM Toy Set; Progressive Trading Magz® 132 Piece Magnetic Building Set containing 84 Short Magnetic Rods and 48 Steel Balls; Logitech® Slim Combo PN 820-008259; Cochlear™ Nucleus® CP810 Sound Processor Coil with 2M Coil Magnet installed; Uigos Digital Kitchen Timer II 2.0.
Codman Certas, SiphonGuard, Bactiseal, Integra, and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries.
©2022 Integra LifeSciences Corporation. All rights reserved. 2523895-1-EN