MicroMatrix® Flex
FOR WOUNDS WITH HARD-TO-REACH AREAS
MicroMatrix Flex is a dual-syringe system designed to enable convenient mixing and delivery of Micro Matrix paste to hard-to-reach wound areas.
Watch the video to learn more!
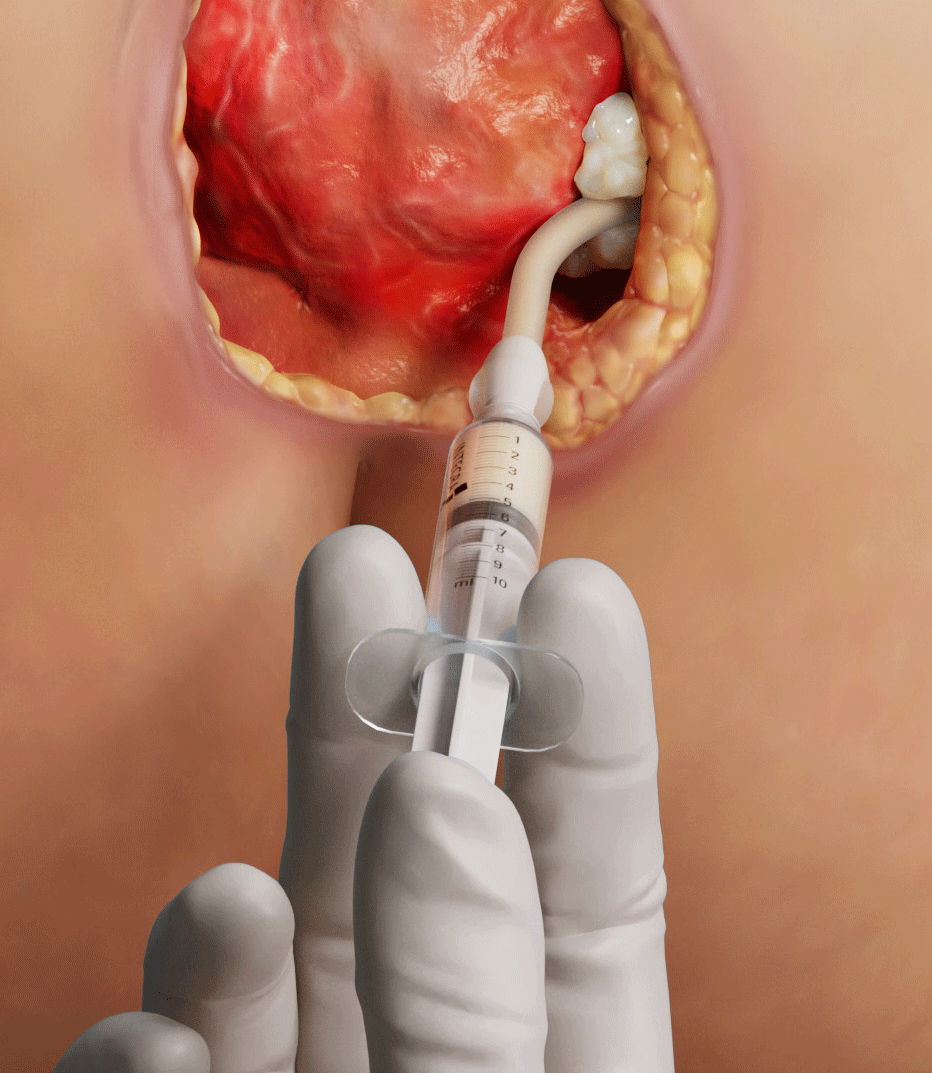
FLEXIBLE TIP FOR THE MANAGEMENT
OF TUNNELED, UNDERMINED OR
IRREGULAR WOUNDS
- Bendable tip allows for ease of application in hard-to-reach areas.
- Tip will hold its shape but can be adjusted during application.
UBM TECHNOLOGY SUPPORTS A QUICK
TRANSFORMATION OF THE WOUND BED1,2
- MicroMatrix is an extracellular matrix derived from porcine urinary bladder matrix (UBM).
- MicroMatrix Flex provides a tool for delivery of UBM technology, which has been shown to support a shift from an inflammatory wound environment to one that facilitates rapid revascularization to support wound closure.2-3
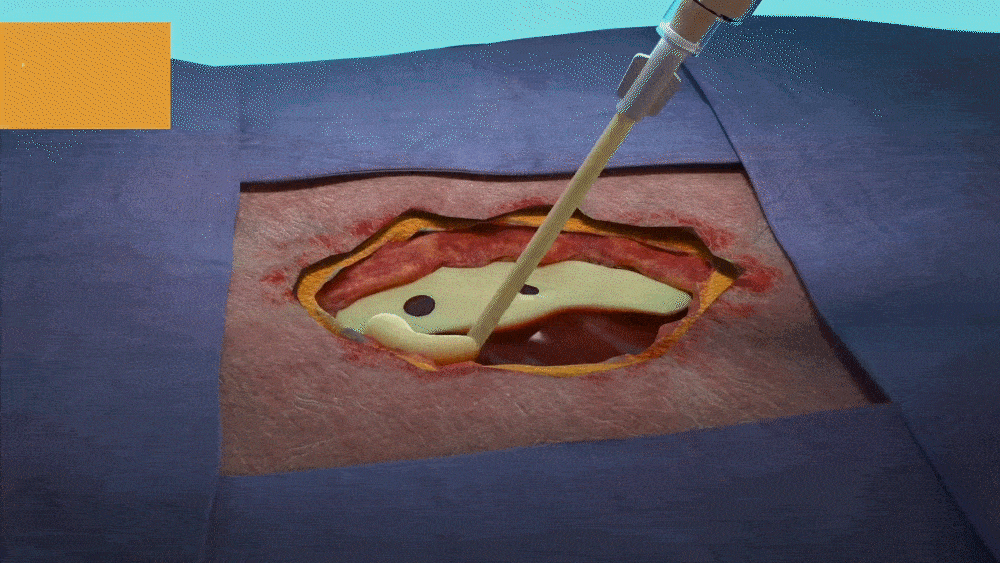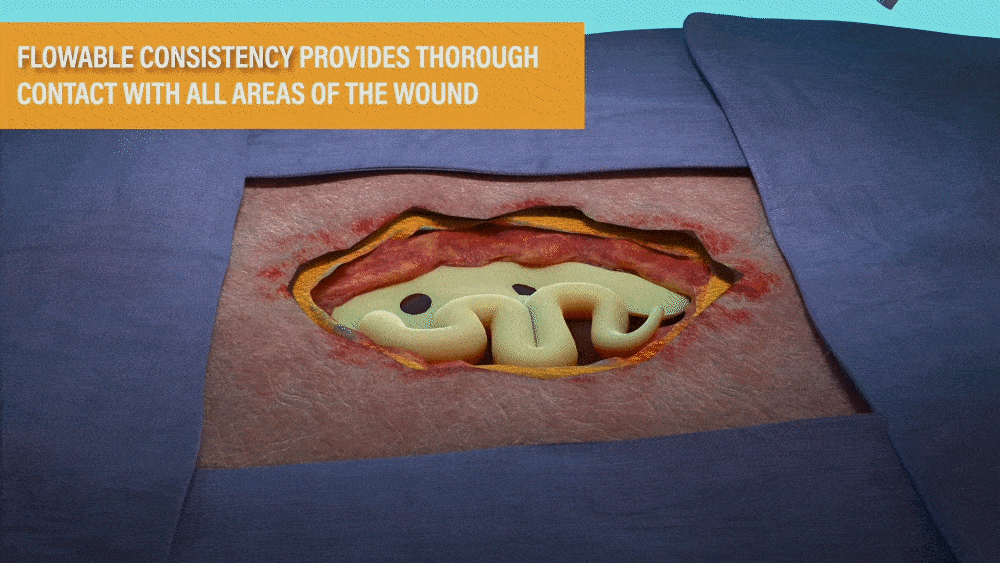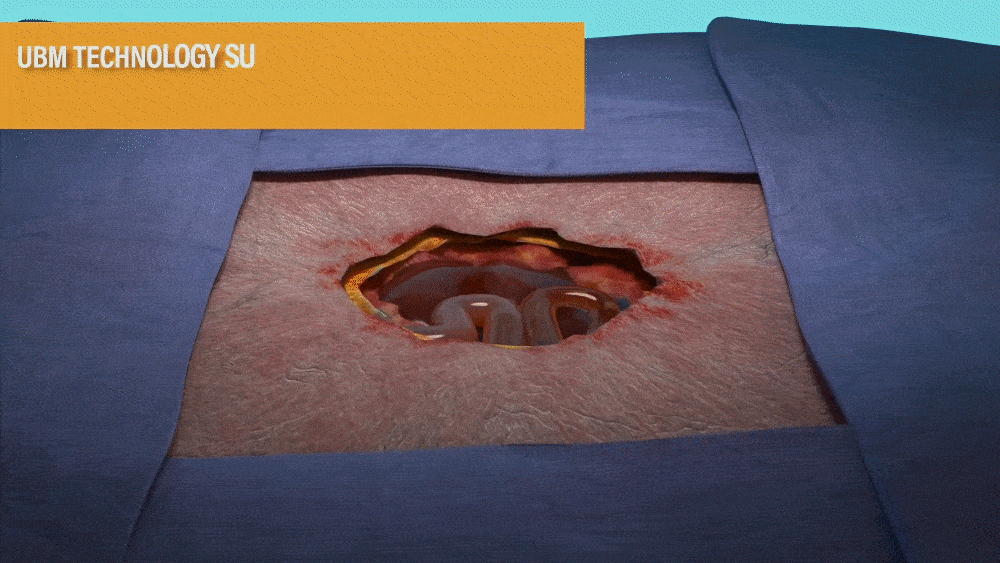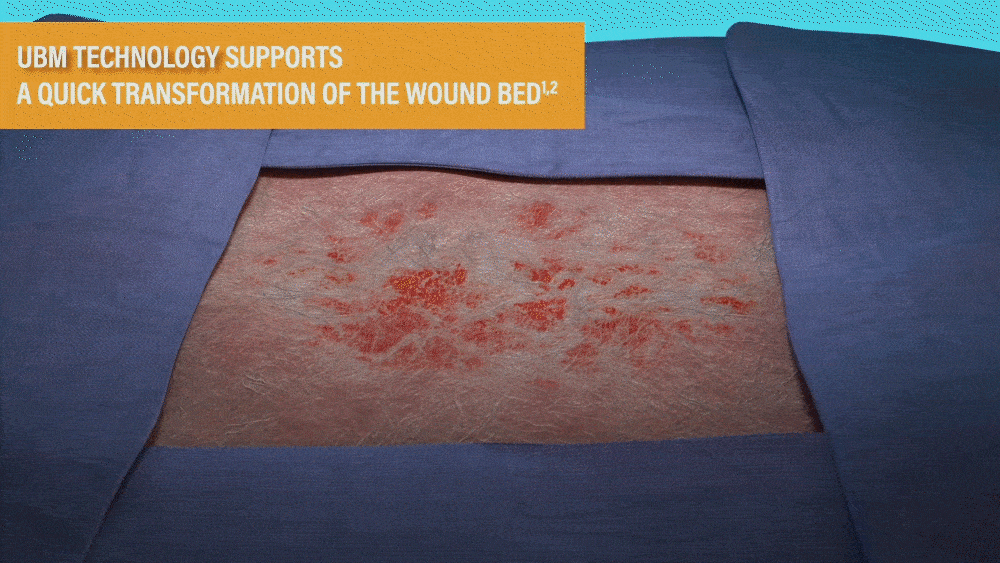
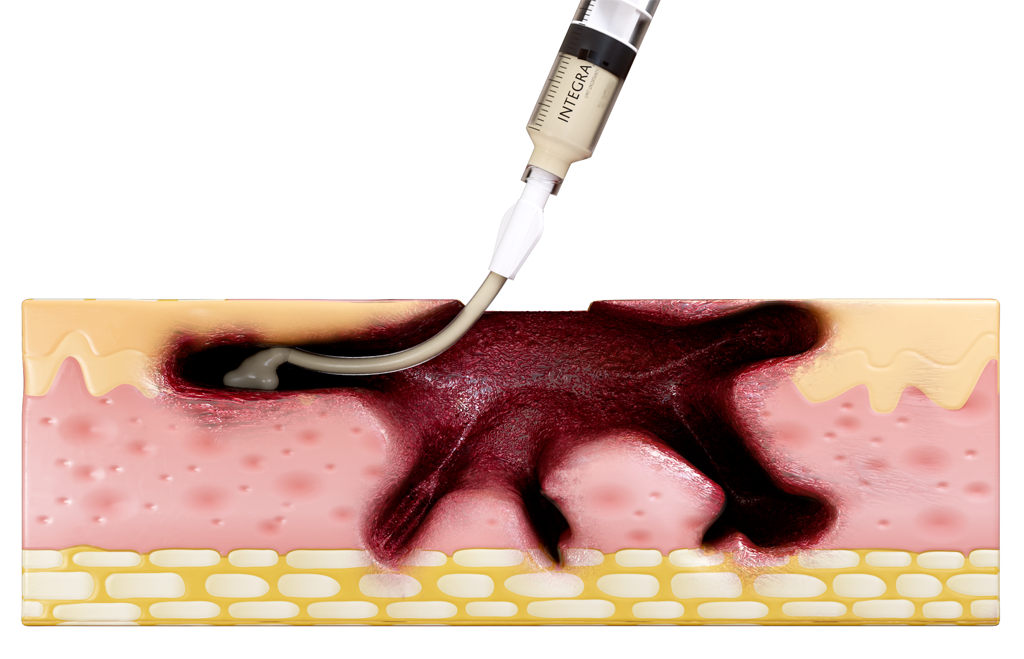
DESIGNED FOR USE IN A RANGE OF WOUNDS WITH HARD-TO-REACH GEOMETRIES INCLUDING:
- Wounds with tunneling and/or undermining.
- Deep wounds.
- Irregular wound beds.
- Wounds against gravity (e.g. bottom of the foot or sacral region).
Contact Customer Service to learn how MicroMatrix Flex® can complement your practice.
MicroMatrix® Flex
INDICATIONS
MicroMatrix Flex is intended for the management of wounds including: partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Moh’s surgey, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, partial thickness burns, skin tears), draining wounds. The device is intended for one-time use.
CONTRAINDICATIONS
- Patients with known sensitivity or allergy to porcine materials.
- Third-degree burns.
WARNINGS
- If active infection is present, treat patient to resolve infection prior to device application.
- Do not use if cracked, broken, or otherwise damaged.
- MicroMatrix Flex is not intended for the treatment of alopecia.
- Do not connect the MicroMatrix Flex device to a hypodermic needle.
PRECAUTIONS
- Always use aseptic technique when handling device.
- Do not re-use.
- Do not re-sterilize.
- Do not cut dispensing tip.
CITATIONS
- Paige JT, Kremer M, Landry J, et al. Modulation of inflammation in wounds of diabetic patients with porcine urinary bladder matrix. Regen Med. 2019;14(4):269-277.
- Brown BN, Londono R, Tottey S, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8(3):978-987.
- Valerio IL, et al. The use of urinary bladder matrix in the treatment of trauma and combat casualty wound care. Regen Med. 2015;10(5):611-622.
