GENTRIX® SURGICAL MATRIX
COMPLEX HERNIA REPAIRS
VENTRAL I PARASTOMAL I HIATAL
Gentrix® devices are biologically-derived, fully resorbable grafts composed of porcine urinary bladder. They are designed for the repair and reinforcement of complex hernia repairs in both open and laparoscopic procedures.
Watch below to learn more!
COMPOSITION & SOURCE
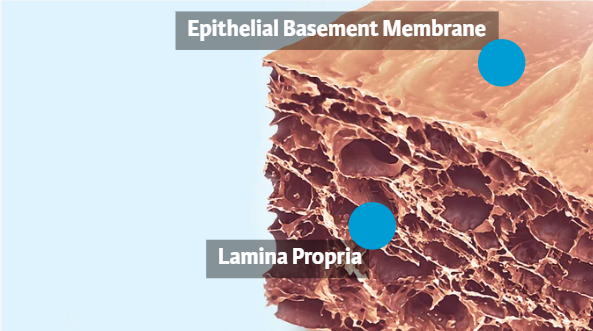
Gentrix devices are derived from porcine urinary bladder. The bladder is harvested and processed so that only the lamina propria and epithelial basement membrane remain.
The epithelial basement membrane can contribute to cell attachment and proliferation.
LAMINA PROPRIA:
The lamina propria surface is conducive for integration of host connective tissue into the scaffold.
EASE OF HANDLING
The flexible design allows the user to easily handle, place and secure the device even in laparoscopic procedures.4-7
Discover the handling properties in a laparoscopic setting in this video
No video selected
Select a video type in the sidebar.
CLINICAL RESULTS8,9
In a recent retrospective study, 64 patients underwent complex incisional hernia repair utilizing Gentrix as a reinforcement graft.9
At a median follow-up of 36 months, the total recurrence rate was 15.6% and no cases of erosion, fistulation, or bowel obstruction were observed.9
Click on the image to access the full manuscript
GENTRIX SURGICAL MATRIX DEVICES
The Gentrix product line is available in multiple layering configurations with sizes ranging from 5x5 cm up to 30x40 cm. It also features a pre-cut, U-shaped graft specific for hiatal hernia repair.
GENTRIX SURGICAL MATRIX THIN
GENTRIX SURGICAL MATRIX
GENTRIX HIATAL
GENTRIX SURGICAL MATRIX PLUS
GENTRIX SURGICAL MATRIX THICK





- 3-layer
- Minimum hydration time: 5 minutes
- Cases could include anastomotic wraps, urethral reconstruction, and small hernias
- Suitable for laparoscopic or open repair
- 6-layer
- Minimum hydration time: 10 minutes
- Cases could include reinforcement of hiatal hernias, parastomal hernias and small ventral hernias
- Suitable for laparoscopic or open repair
- 6-layer
- Minimum hydration time: 10 minutes
- Designed for hiatal hernia reinforcement, featuring a pre-cut shape with smooth, rounded edges
- Designed to be easily handled and secured in laparoscopic surgey
- 8-layer
- Minimum hydration time: 20 minutes
- Cases could include reinforcement of small to midsize complex ventral hernias, inguinal hernias, hiatal hernias, parastomal hernias, and rectal prolapse repair.
- Suitable for laparoscopic or open repair
- 8-layer
- Minimum hydration time: 20 minutes
- Cases could include reinforcement of large complex ventral hernias and abdominal wall reconstruction
- Suitable for open repair
Enter your information to be contacted by a Gentrix representative to learn how Gentrix can complement your hernia practice
ACell® Gentrix® Surgical Matrix Thin
Indications
Gentrix Surgical Matrix Thin is intended for implantation to reinforce soft tissue where weakness exists in patients requiring urological, gastroenterological, or plastic & reconstructive surgery. Reinforcement of soft tissue within urological, gastroenterological, and plastic & reconstructive surgery includes, but is not limited to, the following open or laparoscopic procedures: hernia and body wall repair, colon and rectal prolapse repair, tissue repair, and esophageal repair. Gentrix Surgical Matrix Thin minimizes tissue attachment to the device in case of direct contact with viscera.
Contraindications
Patients with known sensitivity or allergy to porcine materials.
Warnings:
- Device is not intended for transvaginal placement or treatment for pelvic organ prolapse or stress urinary incontinence, bladder support, transabdominal sacrocolposuspension, reconstruction of the pelvic floor, or pubourethral support as device has not been evaluated for these indications and may not provide sufficient support.
- Do not use for high stress applications, such as large, high tension ventral hernias, repair of pelvic organ prolapse, and sacrocolposuspension as the device may not provide sufficient support.
- Device is not intended for bridging hernia defects.
- Exposure to contaminated or infected field can lead to weakening or breakdown of device.
- If active infection is present, treat patient to resolve infection prior to device implantation.
- Do not use if cracked, broken, or otherwise damaged.
Precautions:
- Always use aseptic technique when handling device.
- No studies have evaluated reproductive impact of clinical use of device.
ACell® Gentrix® Surgical Matrix
ACell® Gentrix® Surgical Matrix Hiatal
ACell® Gentrix® Surgical Matrix Plus
Indications
Gentrix Surgical Matrix, Gentrix Surgical Matrix Hiatal, Gentrix Surgical Matrix Plus are intended for implantation to reinforce soft tissue where weakness exists in patients requiring gastroenterological or plastic & reconstructive surgery. Reinforcement of soft tissue within gastroenterological and plastic & reconstructive surgery includes, but is not limited to, the following open or laparoscopic procedures: hernia (e.g. hiatal/ diaphragmatic) and body wall repair, colon and rectal prolapse repair, tissue repair, and esophageal repair. Gentrix Surgical Matrix, Gentrix Surgical Matrix Hiatal, Gentrix Surgical Matrix Plus minimize tissue attachment to the device in case of direct contact with viscera.
Contraindications
Patients with known sensitivity or allergy to porcine materials.
Warnings:
- Device is not intended for transvaginal placement or treatment for pelvic organ prolapse or stress urinary incontinence.
- Device is not intended for bridging hernia defects.
- Exposure to contaminated or infected field can lead to weakening or breakdown of device.
- If active infection is present, treat patient to resolve infection prior to device implantation.
- Do not use if cracked, broken, or otherwise damaged.
Precautions:
- Always use aseptic technique when handling device.
- No studies have evaluated reproductive impact of clinical use of device.
ACell® Gentrix® Surgical Matrix Thick
Indications
Gentrix Surgical Matrix Thick is intended for implantation to reinforce soft tissue where weakness exists in patients requiring gastroenterological or plastic & reconstructive surgery. Reinforcement of soft tissue within gastroenterological and plastic & reconstructive surgery includes, but is not limited to, the following procedures: hernia and body wall repair, colon and rectal prolapse repair, tissue repair, and esophageal repair.
Contraindications
Patients with known sensitivity or allergy to porcine materials.
Warnings:
- Device is not intended for transvaginal placement or treatment for pelvic organ prolapse or stress urinary incontinence.
- Device is not intended for bridging abdominal wall defects.
- Exposure to contaminated or infected field can lead to weakening or breakdown of device.
- If active infection is present, treat patient to resolve infection prior to device implantation.
- Do not use if cracked, broken, or otherwise damaged.
Precautions:
- Always use aseptic technique when handling device.
- No studies have evaluated reproductive impact of clinical use of device.
- Insertion of device through a trocar is not recommended.
- Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Engineering. 2006; 12:519-526.3.
- Sadtler K, Sommerfeld SD, Wolf MT, Wang X, Majumdar S, Chung L, Kelkar DS, Pandley A, Elisseeff JH. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostatis and injury. Seminars in Immunology. 2017; http://dx.doi.org/ 10.1016/j.smim.2017.05.002.
- Data on file (Memo-1108).
- Mehta, A., Afshar, R., Warner, D. L., Gardner, A., Ackerman, E., Brandt, J., & Sasse, K. C. (2017). Laparoscopic Rectopexy with Urinary Bladder Xenograft Reinforcement. JSLS : Journal of the Society of Laparoendoscopic Surgeons, 21(1), eJSLS.2016.00106. https://doi.org/10.4293/JSLS.2016.00106.
- Zografakis, J., Johnston, G., Haas, J., Berbiglia, L., Bedford, T., Spear, J., Dan, A., & Pozsgay, M. (2018). Urinary Bladder Matrix Reinforcement for Laparoscopic Hiatal Hernia Repair. JSLS : Journal of the Society of 2. Laparoendoscopic Surgeons, 22(2), e2017.00060. https://doi.org/10.4293/JSLS.2017.00060.
- Wang, C. Q., Tran, T., Montera, B., Karlnoski, R., Feldman, J., Albrink, M. H., & Velanovich, V. (2019). Symptomatic, Radiological, and Quality of Life Outcome of Paraesophageal Hernia Repair With Urinary Bladder Extracellular Surgical Matrix: Comparison With Primary Repair. Surgical laparoscopy, endoscopy & percutaneous techniques, 29(3), 182–186. https://doi.org/10.1097/SLE.0000000000000611.
- Sasse, K. C., Warner, D. L., Ackerman, E., & Brandt, J. (2016). Hiatal Hernia Repair with Novel Biological Graft Reinforcement. JSLS : Journal of the Society of Laparoendoscopic Surgeons, 20(2), e2016.00016. https://doi.org/10.4293/JSLS.2016.00016.
- Gonzalez C, Russo N, Hanna JP, Tran T, Montera B, Chharath K, Saad AR, Velanovich V. Case–control comparison of separation of component retrorectus urinary bladder extracellular surgical device hernia repair with acellular dermal matrix underlay and prosthetic mesh overlay hernia repair. Int J Abdom Wall Hernia Surg 2021;4:13-9.
- Sasse KC, Lambin JH, Gevorkian J, Elliott C, Afshar R, Gardner A, Mehta A, Lambin R, Peraza L. Long-term clinical, radiological, and histological follow-up after complex ventral incisional hernia repair using urinary bladder matrix graft reinforcement: a retrospective cohort study. Hernia. 2018; doi: 10.1007/s10029-018-1830-0.
Gentrix, Integra and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. Other trademarks are the properties of their respective owners.
2105722-2-EN



