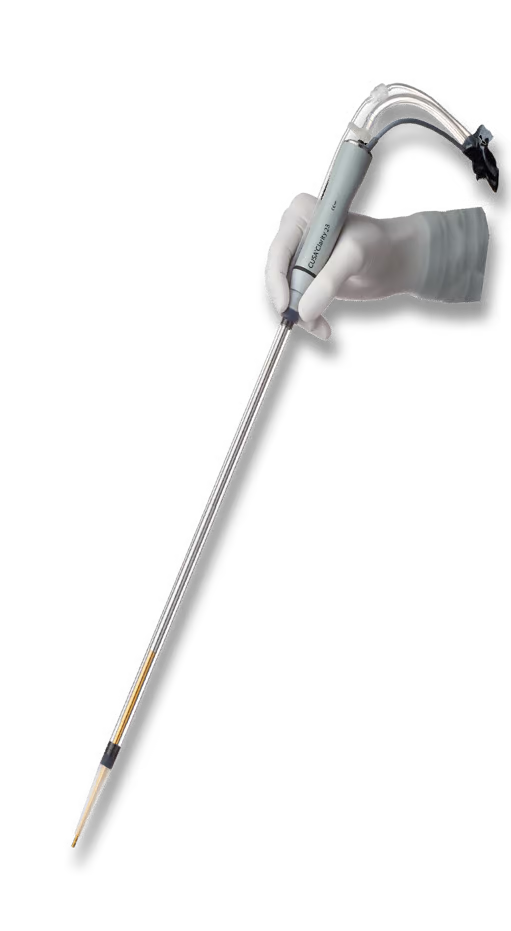
CUSA® Clarity
Introducing the CUSA® Clarity Expanded CEMTM Nosecone
CUSA Electrosurgical Module
Simultaneous Activity
Surgeons can perform simultaneous ultrasonic tissue removal and coagulation on one device!
More Compatibility
Now compatible with a wider selection of Electrosurgical Generators.
Made for CUSA
Designed to be used with CUSA Clarity 23kHz Soft Tissue Tips.
Access The Cusa Clarity Expanded CEM Technical Bulletin
Connect with our Sales Team to learn more about our Laparoscopic Tips
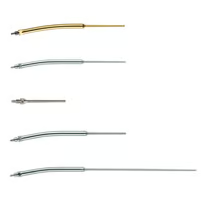
Introducing the CUSA® Clarity Laparoscopic Tips
23 kHz Standard and Extended Length Laparoscopic Tips
Providing 50-75% higher tissue fragmentation rate
The new design provides a 75% higher tissue fragmentation rate using the new Clarity 23 kHz Standard Length Laparoscopic Tip (C7404SEA) when compared to the CUSA Excel+ Laparoscopic tip.1 The new Clarity 23 kHz Extended Length Laparoscopic Tip (C7404LSEA) provides a tissue fragmentation rate 50% higher when compared to the CUSA Excel+ Laparoscopic Tip.1
New Hybrid Flue Reducing Frictional Force
By utilizing a rigid extruded tube material the friction is reduced between the trocar and the flue when compared to the CUSA Excel+ Laparoscopic Tip Flue.2
New Silicone Flue Tip for Secure Positioning
The new silicone flue tip and coupling has been designed to maintain a secure position over the laparoscopic tip during use.

Standard Tip
Understand the mechanics of the CUSA® Clarity and the Magic of Tissue Select®
- Precision and Power Handpieces are designed for safety, speed, and comfort
- Streamlined setup so CUSA® Clarity is ready when you are
- Scalable platform supports future enhancements
Visit our CUSA® Clarity Catalog Pages
Access additional resources and specifications information for the Cusa Brand.
ACCESS THE CUSA® CLARITY GYN BROCHURE HERE.
INDICATIONS
The CUSA Clarity Ultrasonic Surgical Aspirator System is indicated for use in surgical procedures where fragmentation, emulsification and aspiration of soft and hard (e.g. bone) tissue is desirable.
The CUSA Clarity Ultrasonic Surgical Aspirator is indicated for use in: Plastic and Reconstructive surgery, Orthopedic Surgery, Gynecological Surgery and Thoracic Surgery and the following specific uses:
Neurosurgery - including removal of primary and secondary malignant and benign brain and spinal tumors, including but not limited to meningiomas and gliomas
Gastrointestinal and Affiliated Organ Surgery – including removal of benign or malignant tumors or other unwanted tissue, including hepatic parenchyma, in open or laparoscopic procedures, hepatic resection, tumor resection, lobectomy or trisegmentectomy, or removal of tissue during liver allotransplantation and donor hepatectomy
Urological surgery- including removal of renal parenchyma during nephrectomy or partial nephrectomy
General Surgery – including removal of benign or malignant tumors or other unwanted soft or hard tissue in open or minimally invasive general surgical procedures
Laparoscopic Surgery - including removal of hepatic parenchyma in laparoscopic hepatic resection, lobectomy or trisegmentectomy, in laparoscopic donor hepatectomy or laparoscopic cholecystectomy or laparoscopic pancreatic jejunostomy, or pancreatectomy, or laparoscopic appendectomy, laparoscopic colon resection or laparoscopic partial gastrectomy.
CONTRAINDICATION
This ultrasonic aspirator device is not indicated for and should not be used for the fragmentation, emulsification, and aspiration of uterine fibroids.
Please refer to the CUSA Clarity Operator’s Manual for full indications, contraindications, cautions, and warnings.
For use in US only.
REFERENCES:
- Data on File. Clarity Stage 3 Tissue Fragmentation Rate Test Report CC3-LT-DHF-DVR-VR14936, April 2021
- Data on File. Clarity Stage 3 Flue-Trocar Friction Test Report CC3-LT-DHF-DVR-VR14954, November 2021
Corporate Site Privacy Policy Terms of Use
CUSA, CUSA Excel, and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. ©2023 Integra LifeSciences Corporation. All rights reserved. 3245001-EN-2
-1.avif)
.avif)